Details of DPV and References
DPV NO: 411 December 2005
Family: Alphaflexiviridae
Genus: Potexvirus
Species: Pepino mosaic virus | Acronym: PepMV
This is a revised version of DPV 350
Pepino mosaic virus
R.A. Mumford Central Science Laboratory, Sand Hutton, York YO41 1LZ, UK
R.A.C. Jones Department of Agriculture, Baron-Hay Court, South Perth, WA 6151, Australia
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Jones et al. (1980) infecting pepino (Solanum muricatum) plants in Peru. In 1999 the virus was found in tomato (Lycopersicon esculentum) crops in Europe (Van der Vlugt et al., 2000). It subsequently became an important pathogen of tomato in several other continents with considerable international plant health implications. It has filamentous particles of around 510 nm in length that contain one genomic ssRNA molecule (6410 nucleotides). It is transmitted readily by inoculation of sap to many solanaceous species. No vector is known but the virus is easily transmitted from plant to plant through contact.
Main Diseases
PepMV was first discovered in 1974 in two fields of the vegetatively propagated bush fruit crop pepino at Imperial in the Canete Valley of coastal Peru (Jones et al., 1980). Young leaves of infected plants showed a distinct yellow leaf mosaic (Fig. 1) sometimes associated with enations (Fig. 2). The virus was re-isolated from pepino in the region in the 1980's (C. Fribourg, Pers. Comm.), and again in 2000 when it was also found in tomato and wild Lycopersicon spp. (Soler et al. 2002).
In economic terms, the most important disease caused is in tomato, where early foliage symptoms include mosaic, leaf distortion and surface 'bubbling', and plant stunting (Fig. 3). As plants mature, foliar symptoms generally disappear, although isolated yellow spots often appear later in the season (Fig. 4) when they are frequently the only indication of infection on leaves. A leaf symptom occasionally seen is a bright yellow 'aucuba' type marking (Fig. 5). Fruit symptoms include uneven ripening and surface 'marbling' (Fig. 6), which lead to an overall reduction in fruit quality. Economic losses mainly come from its detrimental effect on fruit quality, as its yield impact on fresh weight of fruit is generally minor. Symptoms of the PepMV-induced disease develop more readily in larger fruiting tomato cultivars (beef or classic round-types), while cherry-types are generally unaffected. Environmental factors also influence symptom expression, infected crops sometimes becoming infected without symptoms or only developing fruit symptoms. PepMV-infected plants growing in the glasshouse may become badly scorched under hot summer conditions (Fig. 7) (R. Mumford and C. French, Pers. Comm.). Recent studies suggest that collapse of tomato plants in Spain may be associated with PepMV infection (Soler-Aleixandre et al., 2005). However, this is likely to be due to a synergistic effect with another pathogen as increased damage occurs when plants are co-infected with PepMV and Verticillium (R. Mumford, Pers. Comm.).
Recently, natural-infection was detected in potato (Solanum tuberosum) in Peru by serology with PepMV antiserum (L. Salazar, Pers. Comm.). The virus was detected in plants of cv. Yungay growing in the field in the Andes, and in 14% of the accessions tested in a potato germplasm collection. Infected plants showed a mild mosaic or mottle. In glasshouse tests with the pepino strain, different wild and cultivated potato genotypes developed mild mosaic (Fig. 8), systemic necrosis or symptomless infection (Jones et al., 1980), while the tomato strain caused mottling (Fig. 9) (R. Mumford, Pers. Comm.). However, the precise symptoms caused by the virus in potato in the field are yet to be determined. This is because to date it has only been found occurring naturally in mixed infection with other viruses. In addition to spread by contact transmission, sources of the virus can be introduced to crops by planting infected pepino cuttings or potato tubers (Jones et al., 1980).
Geographical Distribution
First found in one valley in the coastal region of Peru (Jones et al., 1980), but later shown to be present in both coastal and Andean regions of Central and Southern parts of the country (Soler et al. 2002). Since 1999, PepMV has been identified in many European countries (Verhoeven et al., 2003), Canada and the USA (French et al., 2001), and China (Zhang et al., 2003).
Host Range and Symptomatology
The experimental host range is restricted mainly to solanaceous plants (Jones et al., 1980; Verhoeven et al., 2003) but symptomless infections develop in inoculated leaves of Tetragonia expansa (Aizoacaea) and Cucumis sativus (Cucurbitacae). Also, natural infection has been reported in Amaranthus spp. (Amaranthacae), Malva spp. (Malvacae) and Sonchus oleraceus (Compositae) (Jorda et al., 2001; Soler et al., 2002). Differences in symptomatology distinguish the tomato and pepino strains.
- Diagnostic species
- Datura metel and D. stramonium. Both strains cause severe systemic mosaic and, in young leaves, systemic necrotic patching, spotting and flecking (Fig. 10). Inoculated and lower non-inoculated leaves may later develop irregular expanding necrotic patches resulting in generalized leaf necrosis. Young leaves that develop later are infected without symptoms.
- Nicotiana debneyi. The pepino strain causes severe systemic mosaic with some necrotic spots, rings and lines (Fig. 11). Expanding necrotic patches and generalized necrosis of inoculated and lower non-inoculated leaves may develop later, as with Datura spp., especially under shaded conditions.
- N. glutinosa. The pepino strain causes distinct yellowish mosaic on tip leaves 4-5 days after inoculation followed both in older leaves and in leaves produced afterwards by generalised milder mosaic symptoms (Fig. 12). Generalised necrosis of inoculated and lower non-inoculated leaves also develops, as with Datura spp., especially under shaded conditions. With the tomato strain, infection is generally symptomless in inoculated leaves and there is rarely any systemic spread. However, occasionally necrotic and/or chlorotic spots develop in inoculated leaves and there is systemic infection which is either symptomless or consists of chlorotic spots and veinal chlorosis.
- Lycopersicon escultenum. On young glasshouse-inoculated plants, the tomato strain causes systemic mosaic, vein clearing and yellowing appears within 7-10 days post-inoculation (Fig. 13). With the pepino strain, infection is symptomless.
- Nicotiana benthamiana. Systemic mosaic develops with both strains
- Solanum spp. The pepino strain causes mosaic, symptomless systemic infection or severe local and systemic necrotic symptoms depending on the genotype inoculated (Fig. 14). The tomato strain causes mild mosaic.
- Propagation species
- Nicotiana benthamiana, N. glutinosa, N. occidentalis.
- Assay species
- Nicotiana. benthamiana (both strains), N. glutinosa (pepino strain). No local lesion host is known.
- Useful Solanaceous non-host species include petunia (Petunia hybrida) and pepper (Capsicum sinense and annuum).
- Abouhaidar, Xu & Hefferon, in Methods in Molecular Biology, Vol. 81: Plant Virology Protocols - From Virus Isolation to Transgenic Resistance, pp. 131-143, eds. G. D. Foster & S. C. Taylor, Totawa NJ: Humana Press Inc, 1998.
- Aguilar, Hernandez-Gallardo, Cenis, Lacasa & Aranda, Archives of Virology 147: 2009, 2002.
- Cotillon, Girard & Ducouret, Archives of Virology 147: 2231, 2002.
- Dolby & Jones, Annals of Applied Biology 112: 231, 1988.
- Francki, Milne & Hatta, An Atlas of Plant Viruses, Vol. II, Boca Raton: CRC Press, 248 pp., 1985.
- French, Bouthillier, Bernardy, Sabourin, Johnson, Masters, Godkin & Mumford, Plant Disease 85: 1121, 2001.
- Jones, Koenig & Lesemann, Annals of Applied Biology 94: 61, 1980.
- Jorda, Lazaro-Perez, Martinez & Lacasa, Plant Disease 85: 1292, 2001.
- Koenig, Lesemann & Jones, AAB Descriptions of Plant Viruses 350, 1989.
- Krinkels, Prophyta, the Annual May, 2001: 30, 2001.
- Lacasa, Guerrero, Hita, Martinez, Jorda, Bielza, Contreras, Alcazar & Cano, Plagas 29: 489, 2003.
- Maroon-Lango, Guaragna, Jordan, Hammond, Bandla & Marquardt, Archives of Virology 150: 1187, 2005.
- Mumford & Metcalfe, Archives of Virology 146: 2455, 2001.
- Soler-Aleixandre, Lopez, Diaz, Perez de Castro & Nuez, Journal of Phytopathology 153: 464, 2005.
- Soler, Prohens, Diez & Nuez, Journal of Phytopathology 150: 49, 2002.
- Thomas, Mohamed & Fry, Annals of Applied Biology 95: 191, 1980.
- Van der Vlugt, Stijger, Verhoeven & Lesemann, Plant Disease 84: 103, 2000.
- Verhoeven, van der Vlugt & Roenhorst, European Journal of Plant Pathology 109: 419, 2003.
- Zhang, Shen, Zhong, Lu, Chjeng & Li, Acta Agriculturae Shanghai 19: 90, 2003.
Strains
Based upon marked differences in symptoms in hosts such as N. glutinosa and tomato (see Host Range and Symptomatology) and nucleotide sequence (see Genome Properties), two different strains are distinguished:
Pepino strain - defined by the original Peruvian type isolate from pepino (Jones et al., 1980).
Tomato strain - typified by isolates from tomato identified in Europe and elsewhere (Mumford & Metcalfe, 2001; Verhoeven et al., 2003).
The recent finding of genetically distinct tomato isolates in the USA
(Maroon-Lango et al., 2005)
indicates that there could be more than these two strains. However, no biological data has been published to date
on the potentially new ones
(see Genome Properties for more information).
Transmission by Vectors
No vector has been identified. When bees are used for pollination, they can spread the virus within tomato crops (Lacasa et al., 2003). Studies with a common aphid species (Myzus persicae) failed to detect any transmission (Jones et al., 1980).
Transmission through Seed
Seed transmission can occur at low rates (less than one in a thousand), when seed is not properly cleaned before sowing. The virus is external, as it is found contaminating the seed coat and not in the embryo or endosperm (Krinkels, 2001).
Serology
The virus is a moderately good immunogen. High quality polyclonal and monoclonal antisera have been produced, although none that can distinguish between the two strains. A number of them are commercially available.
Relationships
The virus is a typical member of the Potexvirus genus. It is distantly related serologically to Cactus virus X and Narcissus mosaic virus but not to eight other potexviruses (Jones et al., 1980). Nucleotide sequencing has confirmed that while PepMV has the typical genome properties of a potexvirus (see Genome Properties), it is also a distinct species, sharing only limited homology with other members of the genus (Mumford & Metcalfe, 2001; Aguilar et al., 2002; Cotillon et al., 2002).
Stability in Sap
In sap of N. glutinosa, the thermal inactivation point (10 min) was between 60 and 65°C, dilution endpoint 10-4 to 10-5, and infectivity was retained for at least 3 months at 20°C (Jones et al., 1980). Infected tomato sap remained infectious for up to 5 weeks when dried onto various surfaces (e.g. glass, aluminium, plastic) at low temperature and high humidity.
Purification
The following method was used by
Jones et al., (1980):
Mince 100 g infected N. glutinosa or N. occidentalis leaves in 25 ml of a solution at pH 7.8
containing 0.065 M disodium tetraborate, 0.435 M boric acid, 0.2% ascorbic acid and 0.2% sodium sulphite.
Filter homogenate through muslin and centrifuge expressed sap at low speed. To 1 vol. of the supernatant fluid add
0.15 vol. 0.4% silver nitrate and leave at room temperature for c. 3 h. Centrifuge at low speed and
add 4% (w/v) of polyethylene glycol M. Wt 6000 to the supernatant fluid. Leave at 4°C overnight.
Centrifuge at low speed, resuspend the pellets in a solution at pH 7.8 containing 0.065 M disodium tetraborate,
0.435 M boric acid, 0.5 M urea and 0.1% mercaptoethanol. Subject the virus to two cycles of differential
centrifugation and resuspend the final pellets in 0.01 M Tris-HCl buffer, pH 8.0.
A general potexvirus purification method (Abouhaidar et al., 1998) is also used to purify PepMV (Mumford & Metcalfe, 2002).
Properties of Particles
No information.
Particle Structure
The particles are flexuous filaments (Fig. 15), typical of potexviruses and measuring mostly c. 508 x 12.5 nm (Jones et al., 1980).
Particle Composition
Nucleic acid: Linear single-stranded positive-sense RNA. The full nucleotide sequence (6410 bases excluding the 3' poly (A) tail) was determined for two European tomato isolates (Aguilar et al., 2002, Spanish isolate Acc. No. AF484251; Cotillon et al., 2002, French isolate Acc. No. AJ438767).
Protein: In SDS-polyacrylamide gels the coat protein usually migrates as two bands with estimated M. Wt of 26.6 x 103 and 23.2 x 103. The smaller protein is probably a degradation product of the larger one.
Genome Properties
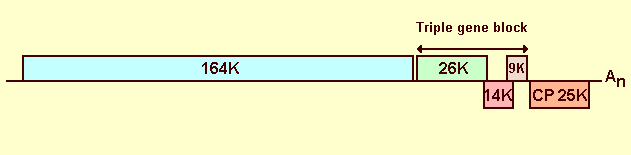
The genomic RNA of the virus is 6410 nt, it has a 3' poly (A) tail and five open reading frames (ORFs) (Mumford & Metcalfe, 2001; Aguilar et al., 2002; Cotillon et al., 2002). ORF 1 (bases 87-4406) is preceded by an 86-base 5' leader sequence; the triple gene block (TGB) consists of three overlapping ORFs: ORF 2 (4432-5136), ORF 3 (5117-5488) and ORF 4 (5340-5594); ORF 5 (5633-6346) is followed by an untranslated region of 64 bases (Fig. 16).
ORFs 1-5 code, respectively, for proteins of M. Wt 164 K, 26 K, 14 K, 9 K and 25 K (coat protein). Homologies with gene products of other viruses suggest that ORF 1 encodes three domains typical of the replicases found in other potexviruses: a methyltransferase domain, an NTPase/helicase domain and a RNA-dependant RNA polymerase domain. ORF 2 also encodes an NTPase/helicase domain. As with other potexviruses, the TGB is thought to have a role in viral cell-to-cell movement
Partial sequence comparisons revealed that the degree of sequence identity is extremely high between tomato isolates, but somewhat lower between the tomato and pepino strains (Mumford & Metcalfe, 2001; Verhoeven et al., 2003). Comparison of coat protein sequences from a range of isolates, found that those from tomato share over 99% identity, but only between 96-97% identity with the type-isolate from pepino in Peru (Mumford & Metcalfe, 2001).
Recently, Maroon-Lango et al. (2005) generated complete genome sequences for tomato isolates PepMV-US1 and 2 (Accession Nos AY509926 and AY509927 respectively) from the USA. The sequence results indicate that these two isolates are genetically distinct from the pepino and tomato strains, as well as from each other. For example, they share only 78 - 83.3 and 80.5 % nucleotide identity with the CP sequences of European tomato strain isolates and between each other respectively.
Relations with Cells and Tissues
In systemically-infected tomato plants, virus can be detected in all tissue types including leaves, stems, roots and fruit. However, the distribution varies with plant age. In older plants, detection in leaves can be difficult and is often only possible in younger leaves. Virus titre in roots varies less. In pepino and potato, the virus is readily detected in leaf tissue but other types of tissues have not been tested.
The intercellular distribution, cytoplasmic alterations and viral inclusions were reported for the pepino strain in N. glutinosa (Jones et al., 1980). Similar studies are absent for the tomato strain.
Ecology and Control
Naturally-infected weed hosts (e.g. wild Lycopersicon spp., Amaranthus spp., Malva spp., Nicotiana glauca, Solanum nigrum and Sonchus oleraceus) have been reported in Spain and Peru (Jorda et al., 2001; Soler et al., 2002). However, the role they play in the epidemiology of the virus is unknown.
In tomato, control is best achieved through ensuring that the virus is excluded from cropping areas both in field crops and in protected cropping. To achieve this, thorough crop hygiene and careful management is essential. Ensuring that growing and fruit packing areas are kept separate is vital. Infections caused by contamination originating from infected fruit being handled on-site in fruit packing houses, is one of the main causes of new outbreaks. Once a tomato crop becomes infected, controlling subsequent virus spread is difficult, due to the ease and speed at which it spreads by contact. Final infection incidences of 90-100% are common. After harvest of infected crops, thorough clean up operations using disinfectants are required to prevent re-infection of subsequent crops. A range of disinfectants are effective against the virus and should be used to decontaminate equipment and surfaces.
In pepino, crop hygiene and management are also important with particular care needed in selecting healthy plants to take cuttings and during handling when transplanting healthy cuttings in the field. In potato, a combination of avoiding planting infected tubers and suitable hygiene measures to avoid contact transmission is needed.
Notes
In addition to PepMV, tomato and potato become infected with two further potexviruses: Potato virus X (PVX) and Potato aucuba mosaic virus (PAMV). While natural infections with the latter are very rare nowadays in potato and tomato, PVX is common in potato and is also sometimes found in tomato crops. As a result, the different potexvirus infections in both crops must be distinguished by either serological (e.g. ELISA) or molecular methods (e.g. PCR). To date, no other potexvirus is reported infecting pepino naturally.
Acknowledgements
In addition to those who kindly supplied photographs, we would like to thank Cesar Friboug, Luis Salazar, Nicola Spence and Chris French for allowing us to use their unpublished data.
Figures

Enations caused by the pepino strain, a symptom sometimes associated with natural infection in leaves of pepino plants.

Early 'nettlehead' symptoms caused by the tomato strain in tomato. Image kindly provided by Kevin Hamilton.

Isolated, yellow spots caused by the tomato strain, a symptom that often appears later in older plants of on tomato.

Bright yellow 'aucuba' type markings caused by the tomato strain, a symptom that develops occasionally in tomato. Image kindly provided by Chris French.

Tomato fruit symptoms showing uneven ripening and surface 'marbling' caused by the tomato strain (left), healthy with normal appearance (right).

Scorch symptoms showing on tomato strain-infected plants growing in the glasshouse under hot summer conditions. Image kindly provided by Nicola Spence.

Expanding necrotic spots and yellowing caused by the pepino strain in inoculated leaflets of potato cv Merpata.

Systemic necrotic spotting, flecking and loss of lower leaves caused by the pepino strain in Datura stramonium.

Typical symptoms caused by the tomato strain in young glasshouse inoculated tomato plants, 7-10 days post-inoculation. Healthy plant on left.